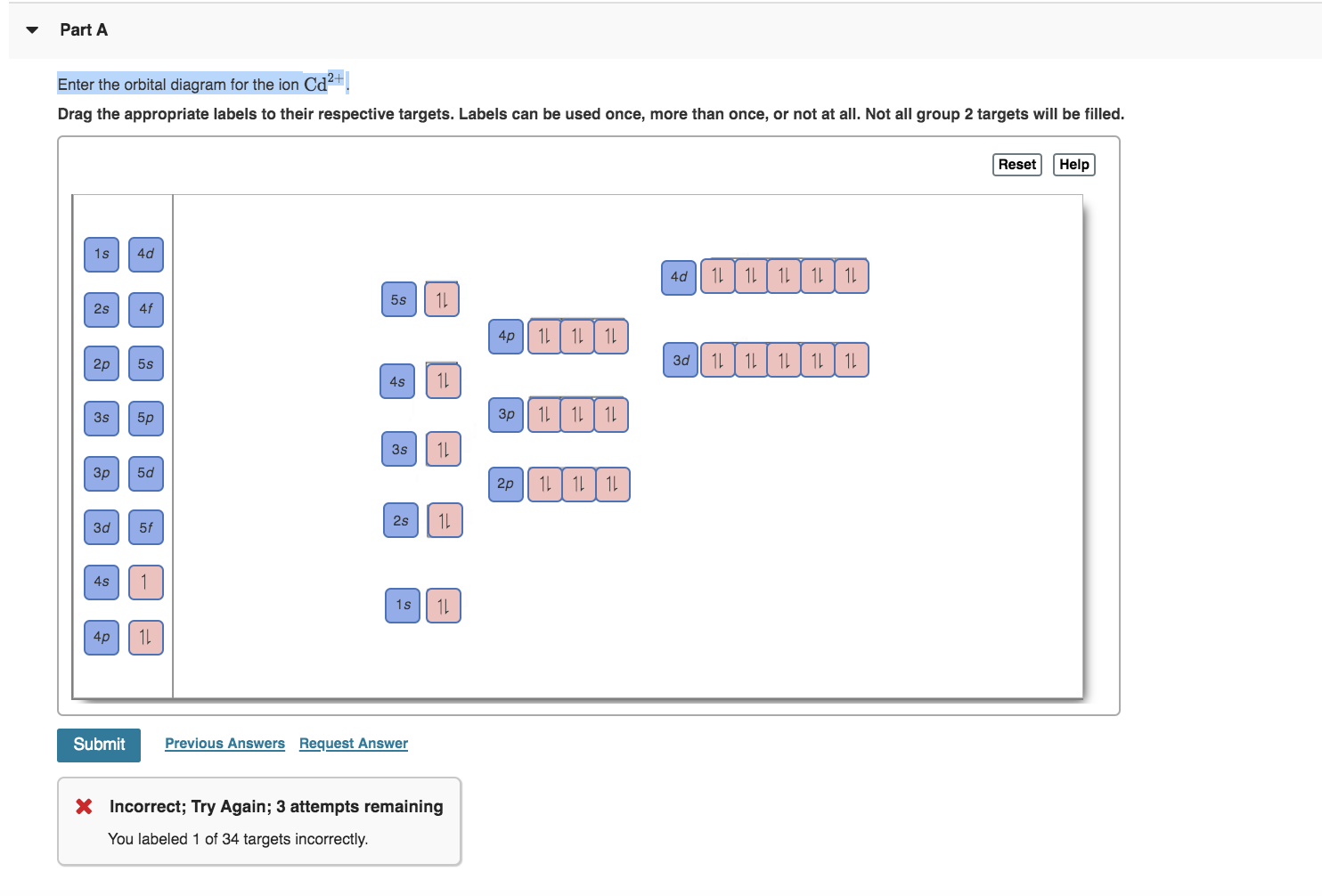
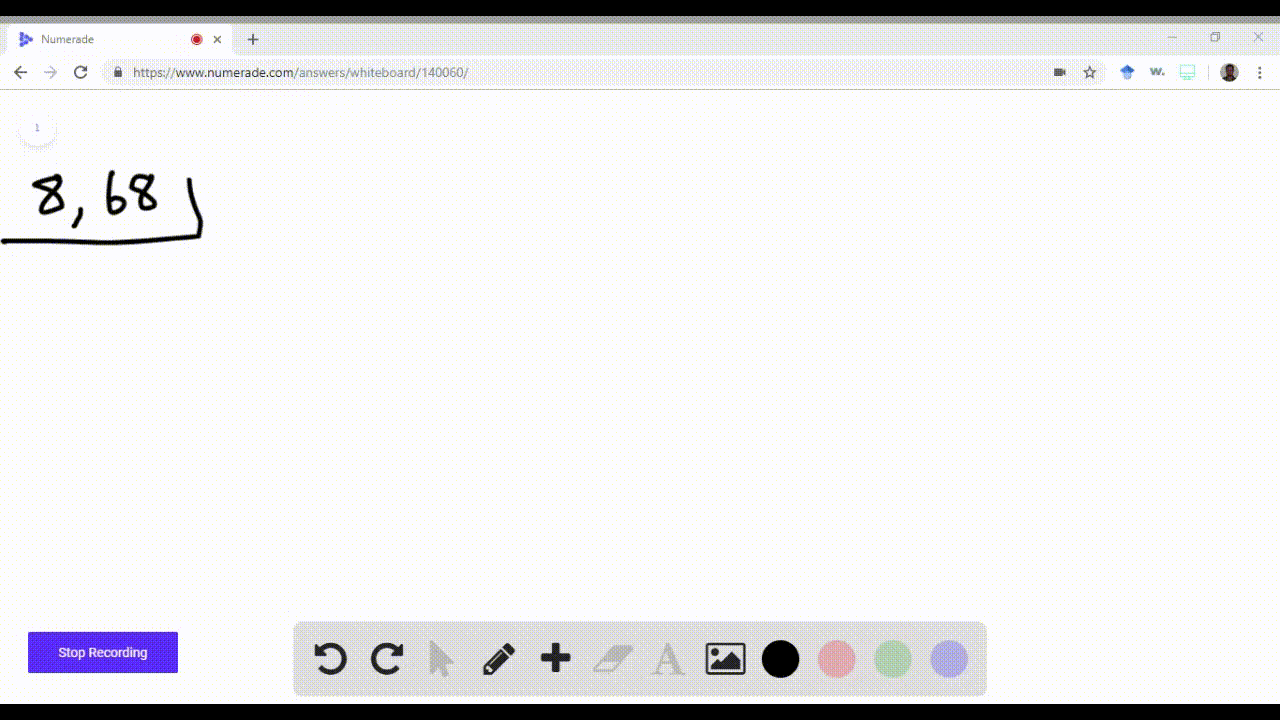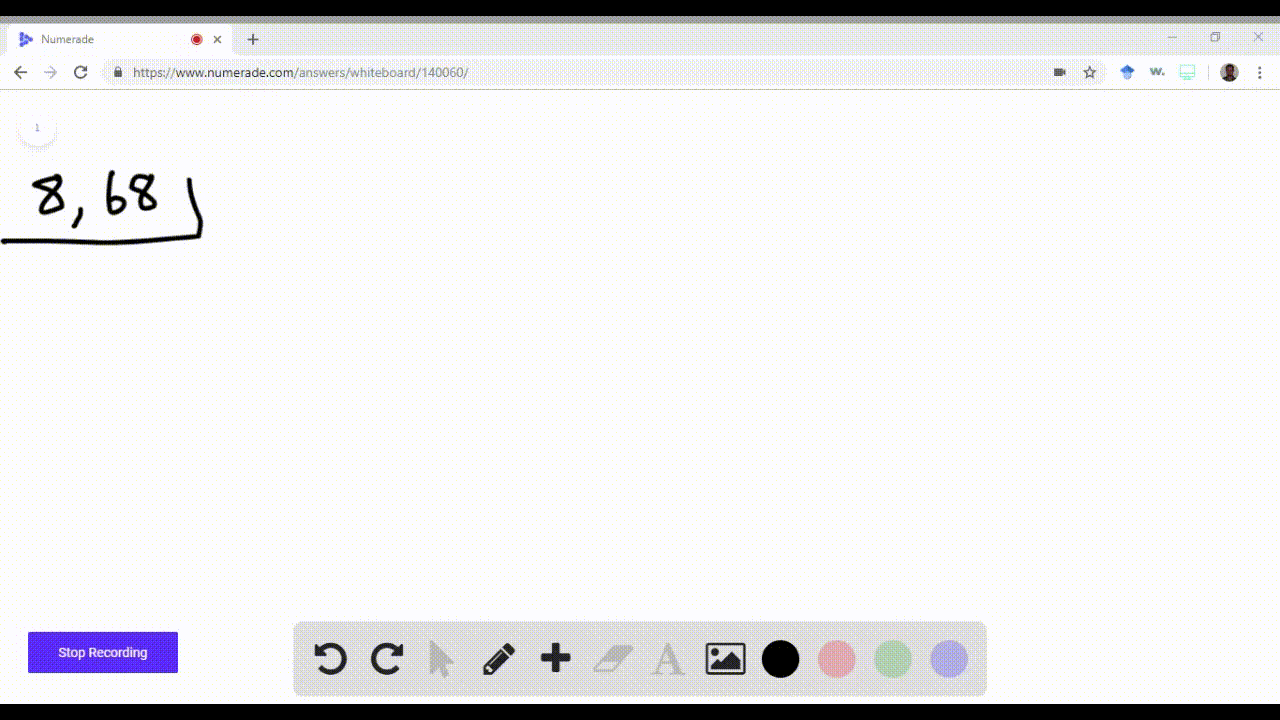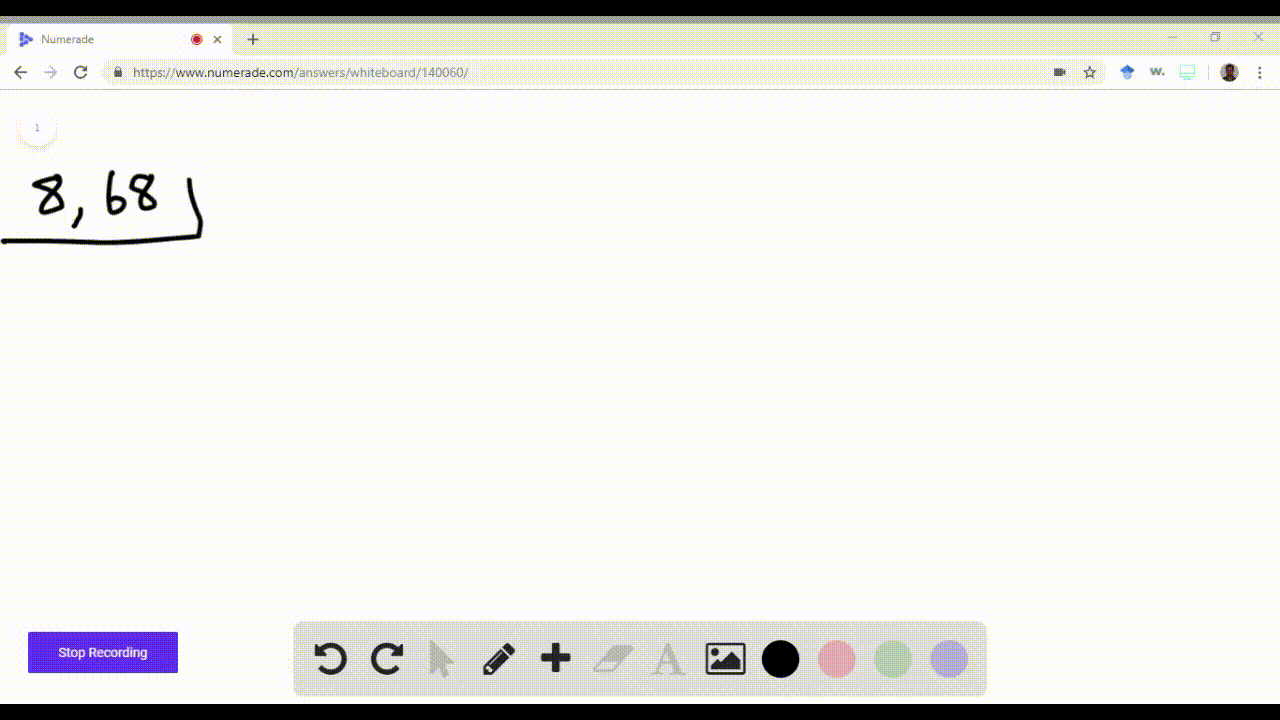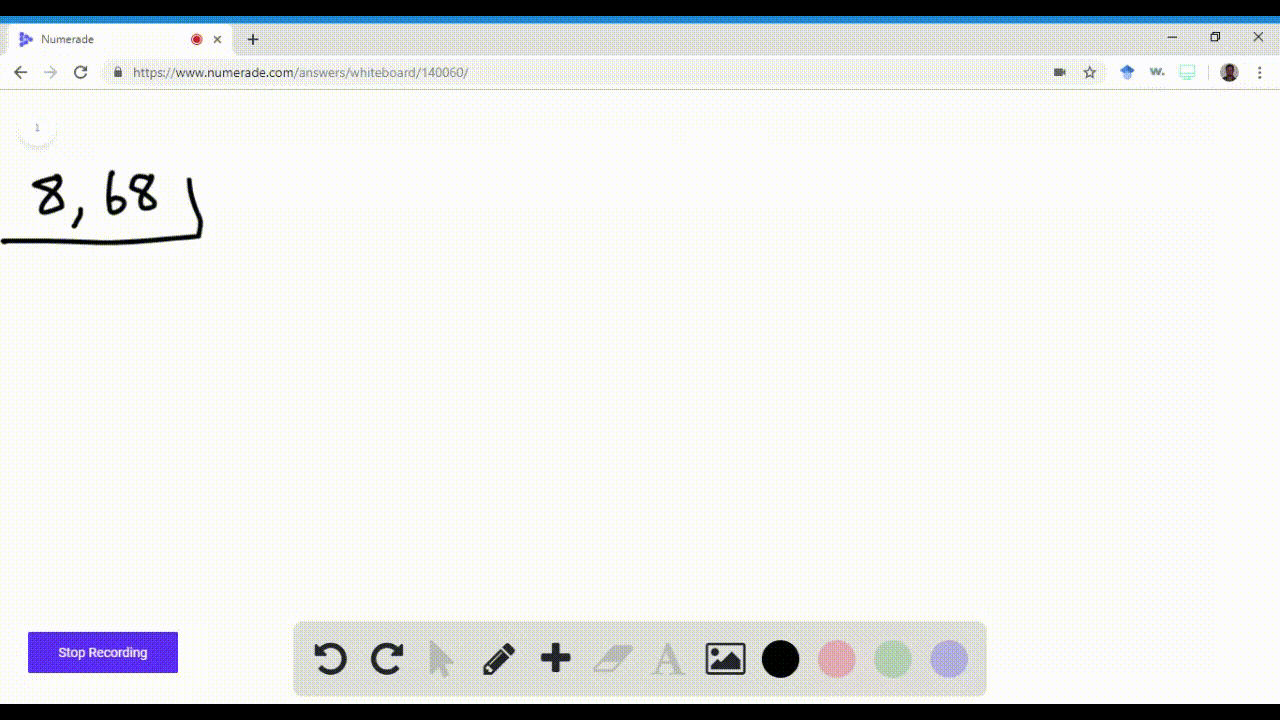
enter the orbital diagram for the ion cd2+
Drag the appropriate labels to their respective targets. Web Electron Affinities Across The Periodic Table The Height Corresponds To Download Scientific Diagram.

Targeted Synthesis Of Carbon Supported Titanate Nanofibers As Host Structure For Nuclear Waste Immobilization
Then the next ten electrons enter the 4d orbital just like the 3d orbital.
. Web Enter the orbital diagram for the ion Cd2 When an element is a cation you REMOVE electrons. Web Answer only. Therefore cation is diamagnetic in nature.
Ground state electron configuration for Au. Web Enter the orbital diagram for the ion Cd2Cd2. Web Click within the orbital to add electrons.
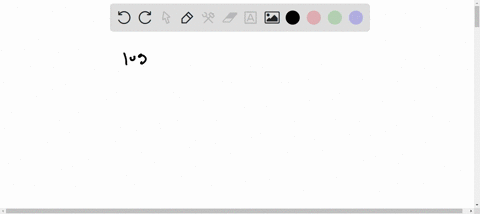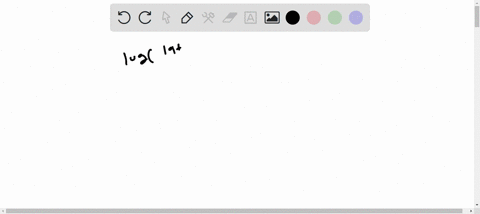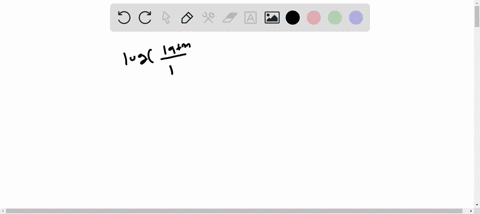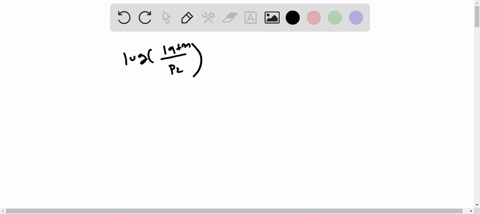
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10. Enter the orbital diagram for the ion Mo3. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10.
So the next two electrons will enter the 5s orbital just like the 1s orbital. So the electron will enter the 4s orbital first and enter the 3d orbital when the 4s orbital is full. Labels can be used once more than once or not at all.
Question write out a balanced net ionic chemical reaction for the. Add them in order of. Web Question Write orbital diagrams for each of these ions.
Electrons are generally removed from the s sub-level 1 Remove two. Not all group 2. Web Enter the orbital diagram for the ion Cd2 When an element is a cation you REMOVE electrons.
Draw orbital diagrams for atoms with the following electron configurations. Use the buttons at the top of the tool to add orbitals. Okay so the first element that has.
Write orbital diagram for each ion and determine if the ion is. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. A Mo Diagram Along With The Orbital Energy Gap In Ev And.
Lets look at the question. Web The atomic number of cadmium Cd is 48. Web The 4p orbital is now full.
Drag the appropriate labels to their respective targets. Web write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. Web Construct the orbital diagram of the F- ion.
Web VIDEO ANSWERHello ribbon. Web Here the energy of 4s orbital is less than that of 3d. Thus the ground state electron configuration of this element is.
Hence the orbital diagram for ion is as follows. So here we are asked to write the electronic configuration of so certain given elements. The method of entering electrons.
Solved Enter The Orbital Diagram For The Ion Cd2 Cd2 Drag The Appropriate Labels To Their Respective Targets Labels Can Be Used Once More Than Once Or Not At All Not All Group
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A Cd2 B Au C Mo3 D Zr2

Solved Part A Enter The Orbital Diagram For The Ion Cd Use Chegg Com
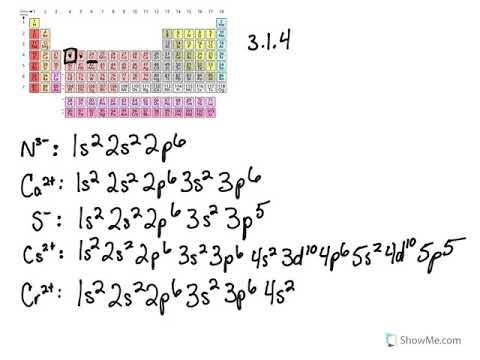
3 1 Electron Configurations Problems Chemistry Libretexts
Adsorption In A Binary System Of Pb Ii And Ni Ii Using Lemon Peels

Solved Write The Full Electron Configuration And Orbital Chegg Com
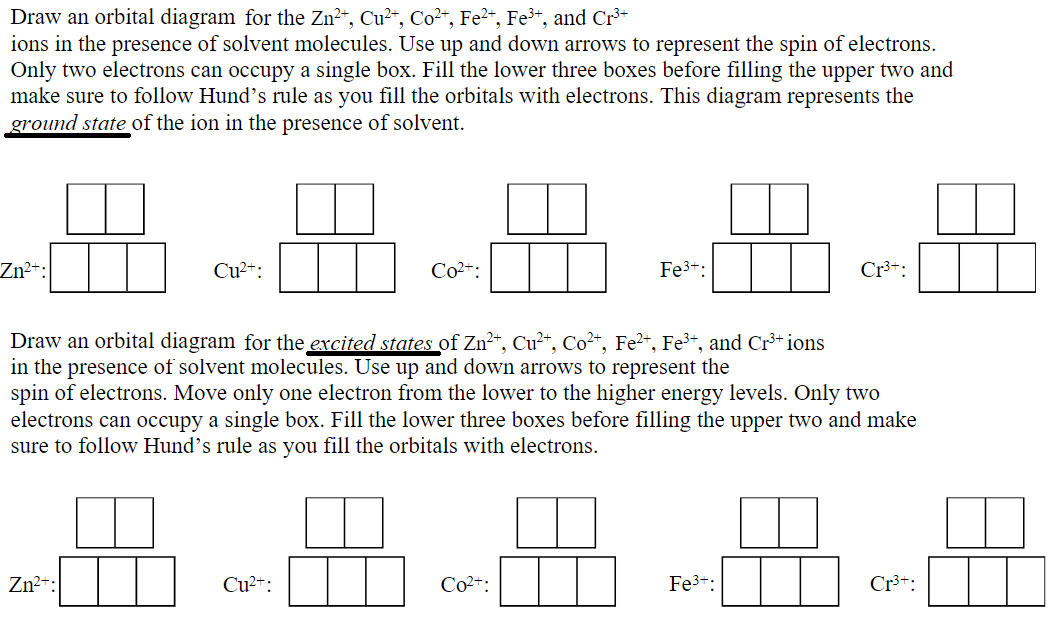
Solved Draw An Orbital Diagram For The Zn2 Cu2 Co2 Chegg Com

Draw The Orbital Diagram For Au Homework Study Com
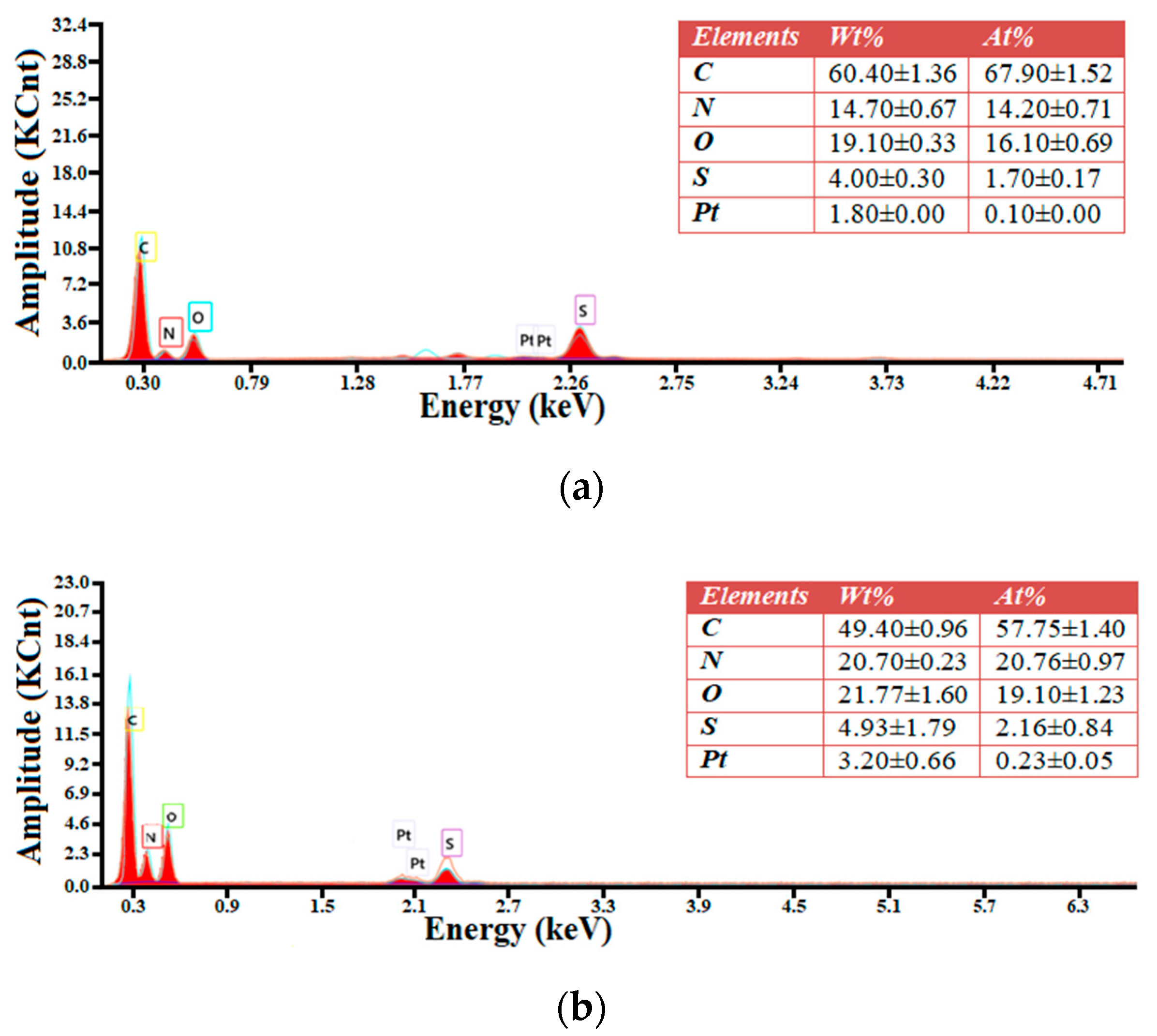
Molecules Free Full Text Oxidized Biomass And Its Usage As Adsorbent For Removal Of Heavy Metal Ions From Aqueous Solutions Html

Webelements Periodic Table Cadmium Properties Of Free Atoms
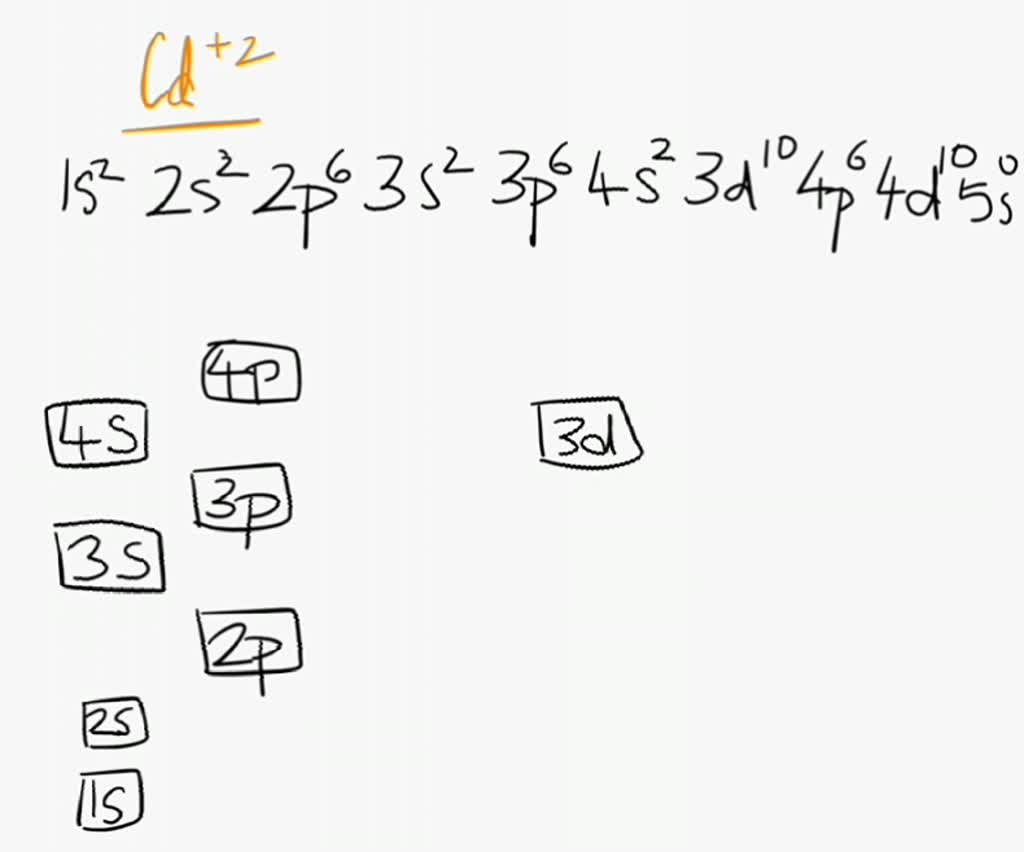
Solved Enter The Orbital Diagram For The Ion Cd2 Drag The Appropriate Labels To Their Respective Targets Labels Can Be Used Once More Than Once Or Not At All Not All Group

Aggregation Adsorption And Morphological Transformation Of Graphene Oxide In Aqueous Solutions Containing Different Metal Cations Environmental Science Technology
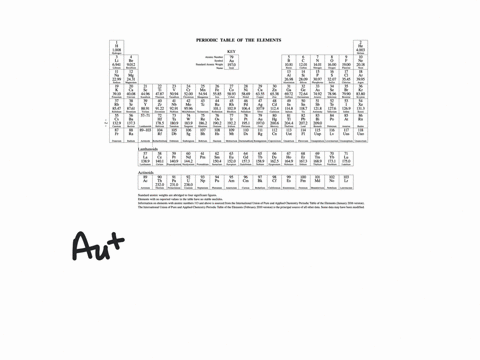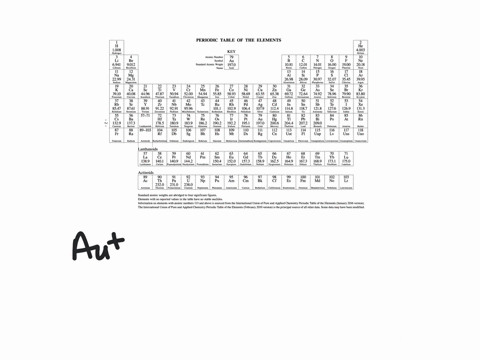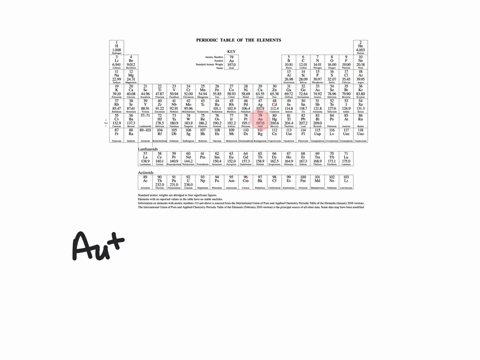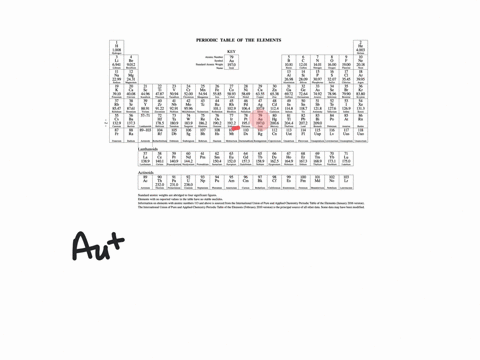
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A Cd2 B Au C Mo3 D Zr2

How To Write The Electron Configuration For Cd And Cd2 Youtube

Targeted Synthesis Of Carbon Supported Titanate Nanofibers As Host Structure For Nuclear Waste Immobilization

How To Write The Electron Configuration For Cd And Cd2 Youtube
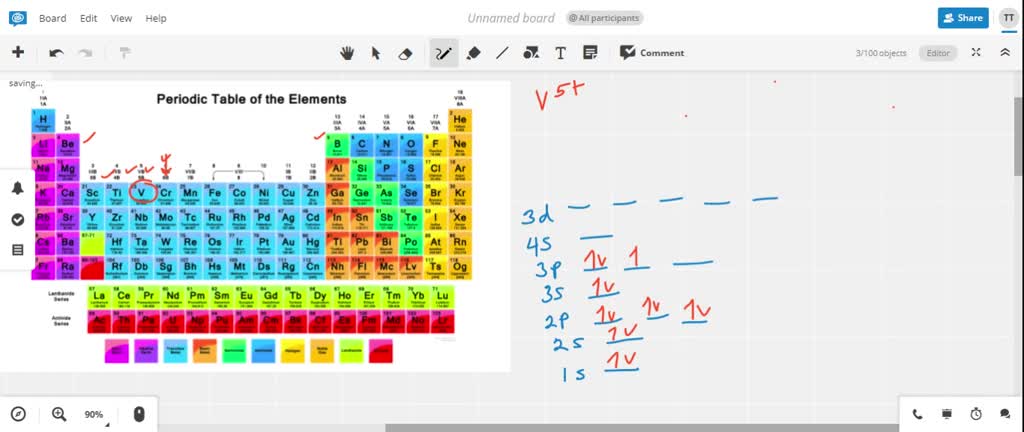
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2 D Fe3